As an integrated industrial hemp company, owning the entire supply chain from seed to sale of finished products, we are constantly trying to keep up with a changing regulatory landscape. There are hints that regulatory requirements will be finally announced so thought it timely to review current rules impacts of regulations on the hemp industry. The number of government organizations that influence the hemp industry is numerous including the United States Department of Agriculture (USDA) and Food and Drug Administration (FDA) to name a few. Navigating between three- and four-letter acronym agencies is no mean feat! Varying guidance or delays in regulatory decisions by both state governments and federal governments make it difficult to smoothly operate in this market sector. Seemingly small changes such as one state changing label requirements for hemp-based products creates operational complexity and cost increases. The uncertainty in regulation has major impacts (other than company headaches) on the hemp industry market potential which has been projected to be greater than $20B in a mere three years (Yahoo! Finance, Figure 1).
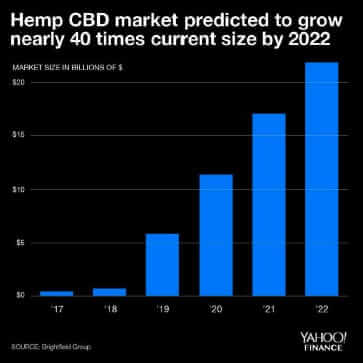
Figure 1: US Hemp CBD Market Forecast by year
There is a need for regulatory consistency for the hemp industry. Providing consistent guidance will allow farmers to determine the risks involved with growing hemp that include compliance as well as costs and profitability. Consumer product quality remains a very large issue with CBD products where over 75% products sold today do not meet potency requirements and may have THC levels beyond 0.3% (Bill J. Gurley, University of Arkansas, June 2019 presentation to the FDA; Rosemary Mazanet, Columbia Care, June 2019 presentation to the FDA). In addition to quality issues, CBD has been claimed to cure as many as 115 different conditions, some may be valid, others are wishful thinking. FDA guidance on how phytocannabinoid rich hemp products should be considered (food, supplement or drug) will provide standards on how products are produced, quality testing standards, consistent labelling requirements, as well as determine how research should be conducted to substantiate health claims.
Colorado has taken a unique approach of bringing together public and private stakeholders to modify the state regulatory environment through the CHAMP initiative (Colorado Hemp Advancement and Management Plan). The CHAMP initiative evaluated the entire life cycle of the industrial hemp industry from seed to banking and marketing. While the full report and recommendations for regulations or legislation is due in Q2 2020, and will be discussed when the reports are published, it is important to understand that the CHAMP meetings have allowed progressive responses to proposed federal regulation.
USDA: Proposed hemp growing regulations and the Interim Final Rule
Colorado, and other states, have worked for the past five years under the regulatory system enabled by the 2014 Agriculture Improvement Act, also known as the Farm Bill. In this bill, hemp was allowed to be grown under a pilot program with guidance on how the states should regulate the growth of hemp. The 2018 version of the Farm Bill effectively moved hemp and hemp-based products out of a pilot phase and called for the USDA to develop a final regulatory plan for farming industrial hemp. The initial USDA plan termed the Interim Final Rule (IFR) was published with a request that individual states provide comments and submit their own plans on how they would comply with USDA regulations. Understanding the implications of how the regulations proposed in the IFR will affect hemp growers is extremely important. In Colorado’s response to the USDA IFR, Colorado has asked the USDA to extend the 2014 growing rules for another year to ensure better understanding of the proposed rule changes and arrive at a balance between protecting public health and healthy hemp farming industry. Quite frankly, it is disappointing that states without significant numbers of hemp farmers have adopted the IFR as written without critical consideration of impact. To directly quote a Colorado state senator, “The regulations proposed in the IFR will kill the hemp industry.” Colorado, being the largest producer and having the longest tenure in growing hemp has assembled a very thoughtful and data driven response to the IFR. Two major highlights of the plan and why suggested changes be made to the IFR are listed below. The link to Colorado’s response to the IFR is located here and will provide a more detailed and complete explanation of the impact to the IFR and what changes are suggested. Note that the response to the USDA was supported by the Colorado State Senate resolution 20-005 with overwhelming support.
- Sampling periods and coverage.
The IFR calls for 100% of the registered hemp fields be tested at DEA approved laboratories prior to harvest. In addition, the crop is to be completely harvested 15 days after sampling. Please note that the sample to determine the tetrahydrocannabinol (THC) content in the plant takes the flower from the top 2-6” of the plants free of stem, leaves and seed constitute the tested sample. This will have the highest concentration of cannabinoids in the plant. Additionally, there is a 15 day period from when the sample is submitted to when the fields must be harvested with harvest prior to receiving test results prohibited.
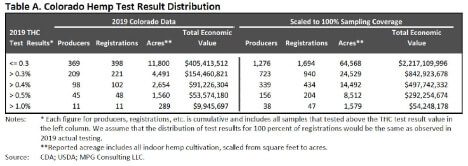
In 2019, Colorado had 87,000 acres registered to plant hemp through 2,600 growers. Under
Colorado’s state plan, harvest can occur within a 30 day window after sampling. Employing a random sampling Colorado 23% (619 registrants, 2,712 lots) were evaluated. Using the proposed IFR rules would create a large bottleneck and resource constraint where the state laboratory would need to expand by at least four-fold to meet testing demands and seek DEA registration for hemp laboratories. Needless to say the IFR rules would create delays in the time farmers could harvest which puts more risk on the crop due to weather variations, especially in Rocky Mountain states.
Colorado has recommended that there be a 30-day period between sampling and harvest, consider random sampling, and eliminate the DEA laboratory requirement so that state laboratories with appropriate standards (ISO17025) may continue testing for crop compliance.
- Thresholds for Destruction and Negligence.
Since 2014 Colorado has destroyed over 3360 acres of hemp worth roughly $115,000,00, in total, due to the hemp testing above 0.3%. If the IFR requirements of 100% testing at the 0.3% level were implemented for the 2019 harvest, the estimated acres that would need to be destroyed is estimated at 24,500 with an economic value of $842,600,000.
Colorado has proposed several possible methods for helping avoid destruction of these crops that protects the industry against bad actors yet allows use of crops that test above 0.3% in the sampling period. As stated above, the sampling takes the uppermost flower from the plant which will have the highest cannabinoid concentration. Three simple changes would be to allow post-harvest testing, increase the destruction threshold to 1% and allow non-compliant hemp to enter a THC-remediation program where extractor/processors would remove THC from the extracted oil so that only 0.3% THC products would then be able to be sold.
Typically, hemp is sold to processors as a whole plant homogenate that is a mixture of flowers, leaves and stems. Since the leaves and stems of the plant contain very small amounts of cannabinoids, a plant that tests slightly higher than 0.3% would be compliant when turned into homogenate. Elevating the threshold and requiring any crops that still test higher than 0.3% and <1% to enter a remediation program at reduced costs allow the farmer to recover costs and ensures that deviate from industrial hemp definitions be commercialized.
Implementation of these changes would have led to 1% of Colorado’s hemp crop to be destroyed for non-compliance.
Setting the threshold at 1% THC for negligent violations verses 0.5% would mitigate grower risk due to some uncertainties in crop growing conditions, and allow a more efficient industry. Under the IFR rules, 48 negligent violations would be issued, as opposed to 11 if a 1% threshold was adopted. Non-compliant hemp, regardless of the threshold set, should be allowed to be destroyed by the farmer on-site under the supervision of state or tribal agriculture departments.
Moving this threshold balances the need to protect public health and facilitate the development of a thriving hemp industry.
FDA: Is CBD safe; should industrial hemp oil be a food ingredient, dietary supplement or drug?
As clearly stated in the 2018 Farm Bill the FDA has oversight of products derived from industrial hemp. Although there have been many meetings and conversations by the FDA and the public, progress is moving at a glacial pace. The new commissioner, Stephen Hahn understands that the toothpaste is out of the tube so the situation needs to be managed: “We’re not going to be able to say you can’t use these products. It’s a fools errand to even approach that [.] We have to be open to the fact that there might be some value to these products and certainly Americans think that’s the case. But we want to get them information to make the right decisions.” The FDA just opened the public comment and invitation for submission of data for clarifying the points listed below, but has yet to lay out a definitive pathway for regulation of CBD-containing products. The March 2020 report to the congressional appropriations committees provides insight as to the FDA focus and goals for the coming year. While the FDA is exploring how various CBD products can be lawfully marketed, concerns have been expressed regarding product safety, mislabeling products, and that the products contain contaminants such as THC, heavy metals and pesticides as there are no manufacturing standards. The FDA recognizes that research has been restrictive until the farm bill passed so is inviting all data submissions from public and private entities, reaching out to state health departments as well as ensuring there are incentives for performing clinical research. It is critical that the industry respond to the FDA’s request so that researchers can design studies to answer those questions definitively. What has been needed is exactly what concerns the FDA has so that researchers can design studies to answer those questions definitively.
The key questions the FDA is seeking to address:
- What happens if you use CBD daily for sustained periods of time?
- What level of intake triggers the known risks associated with CBD?
- How do different methods of exposure affect intake (oral, topical, smoking or vaping)?
- What is the effect of CBD on the developing brain?
- What are the effects of CBD on an unborn child or breasted newborn?
- How does CBD interact with herbs and botanicals?
- Does CBD cause reproductive toxicity in males?
- Are there different safety concerns for use in certain animal species, breeds or classes?
- Are any residues formed in edible tissues of food producing animals.
In addition to these questions, the FDA is seeking more information on identity standards on varying CBD products such as full-spectrum or broad-spectrum oils and how these products are derived. A grant has been awarded to study CBD effects on fetal growth in pregnant women, and an initiative has been launched with the University of Mississippi to sample commercial CBD products to determine CBD and THC levels in 100 cosmetic products as well as dermal penetration that is intended to be published in August, 2020.
In the 2020 report, the FDA reiterates priorities focused on determining safety, enforcement of products making disease claims, and ensuring product potency and purity. Currently the FDA has stated that it is illegal to introduce CBD into human or animal food, market as a dietary supplement, and CBD may be illegal in cosmetics if intended to affect structure/function or address a disease. Minor cannabinoids CBG and CBN have been mentioned as well in regard to concerns about claims and safety. It is very important to note that the FDA considers CBD a schedule V drug due to clinical trials beginning on Epidiolex prior to CBD being marketed. While these statements have been repeated by the FDA over the past three years there is cause for optimism for hemp growers, manufacturers and consumers. The FDA is actively considering potential pathways for certain CBD products to be marketed as dietary supplements. Additionally, the report states that the FDA has the authority to create an exemption through notice and public comment.
While the report gives better insight as to the focus and plan moving forward, it is very puzzling on why more progress has not been made. As stated above, the main focus of the FDA is whether these compounds are safe. One could argue that there is a wealth of information that can be compiled to answer these concerns with any additional questions being addressed by specific studies with defined timelines. In contrast to the glacial pace of FDA progress, CBD regulatory pathways have been established in the UK, Germany, and CBD has been approved by the World Health Organization (WHO).
There have been 226 CBD clinical studies registered with the federal government, with over 80 studies completed. These studies have administered CBD to patients at doses as high as 40 mg/kg/day. Each study reports on clinical efficacy as well as side effects. Since one requirement to enter into a clinical study is safety, it is puzzling how these clinical studies could be approved, yet there are so many safety concerns stated in the 2020 report. In a 2018 report WHO states that CBD is generally well tolerated with a good safety profile as well as not demonstrating any public health related problems. The report states that at common consumption levels CBD is fundamentally safe. The Food Standards Agency (FSA), the agency that regulates CBD in the United Kingdom, has approved CBD at a 70 mg per day serving unless under medical supervision. The CBD products must contain <0.01% THC and have no other controlled cannabinoid such as Tetrahydrocannabivarin (THC-V). The FSA does state that they wish more safety data existed for CBD effects in pregnant women and drug-drug interactions, but this agency has laid out specific regulatory requirements with all producers to comply by March 31, 2021. Germany has followed suit allowing CBD to be a food ingredient. This raises the question on why the FDA has not taken a similar approach?
Due to the lack of significant progress by the FDA, both public and private groups are working to obtain regulatory clarity. At the federal level, HR5587 is a bill submitted to the house of representatives by congressman Peterson (MN) to put into law that CBD is an exception to the IND rule stated above and that this product be regulated as a dietary supplement. Utah has issued a resolution SCR11 that “Urges the Issuance of Federal Guidelines to Protect Consumers of Cannabidiol Products” which has been sent to the President and leaders of the house or representatives and senate. Private initiatives are forming coalitions to engage the FDA to obtain progress. Validcare has launched an industry-sponsored CBD liver safety study with a goal of monitoring chronic CBD usage at varying doses on liver function. This study will enroll up to 2000 patients with data planned to be reported in September, 2020. The National Industrial Hemp Council is forming a Consumer Protection Task Force comprised of over 20 subject matter experts to focus on outstanding issues stated by the FDA and engage the FDA’s CBD working group. This approach may be very productive to address specific issues to responsibly accelerate progress towards how CBD will be regulated.
The Impact of the Lack of Regulatory Framework
As stated in the opening of this document, the rules or lack thereof, create a maze of regulations that changes constantly yet needs to be navigated to produce quality products and meet consumer demand. It may seem like an oxymoron pairing the need for regulatory rules with economic success, but the reality is that the lack of clarity by the FDA has stifled the economic potential of the hemp industry by allowing consumer confusion and in effect restricting retail markets. Similarly, not having a usable framework for growing hemp and processing into oil as a manufacturing ingredient has had a negative impact on farmers.
Both the FDA and private companies need to reach an agreement on standards of identity. The definition and criteria for various preparations of CBD vary within the industry. Consumers do not understand what a full-spectrum, broad-spectrum, or isolate product is and what benefits or risk they may obtain with each product. Likewise, there are not uniform manufacturing standards to which all CBD products are held. The result is that many products do not meet potency or purity standards required by the food or dietary supplement industries. The result of confusion and inferior products on the market create mistrust by consumers which may cause significant long term damage to the reputation of this nascent industry.
There has been an expectation that following the passing of the 2018 farm bill that clear regulations would be provided on how hemp would be grown, products would be manufactured, labelled and sold to consumers. Consumers understand that there are numerous health benefits provided by CBD but manufacturers do not know how they should label products or educate consumers without receiving a warning letter from the FDA. Likewise, retail outlets have held off on carrying CBD products as they need to understand where these products should be placed, how they can market, and do not want to face product confiscation or recalls. The lack of clarity has significantly impacted CBD sales. Financial forecasts as shown in Figure 1 has projected the US CBD market to exceed $20 billion dollars by 2022. The current 2020 CBD revenue projection is hopefully going to reach 20% of forecast as shown in Figure 2.
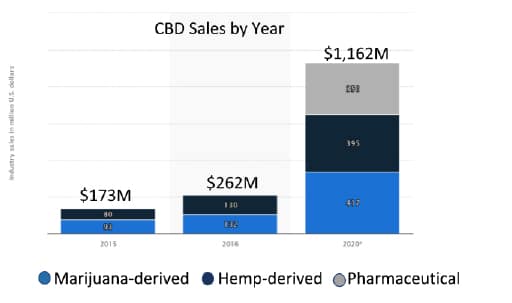
Figure 2: Actual US CBD revenue and projected 2020 revenue by CBD source
The hardest hit with the market not reaching anywhere close to expectations are the hemp farmers. Since hemp is grown like any other commodity, acres grown are planned in the early summer with the hope and expectation that the crop will be purchased at reasonable market rates. Hemp is normally sold at dollars per percentage point per pound (PPPP). Thus, a 10% CBD crop sold at $5 PPPP would be $50 per pound. The 2018 crop production met existing demand with a range of pricing from $3.5 PPPP to $7.5 PPPP. Heading into the 2019 growing season farmers scaled up production that exceeded usable demand causing hemp prices to plummet. Without clarity the anticipated demand was not realized.
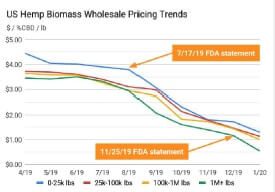
Figure 3: FDA statement correlations to US Hemp prices
Source: Hemp benchmarks: https://hempbenchmarks.com
It is striking to map hemp prices following FDA statements as shown in
Figure 3. Clearly, prices realized by the farmers correlates to continued lack of clarity by the FDA.
Concluding remarks
Clearly the emerging regulations, or lack of direction is causing market confusion that is stifling market growth. Farmers are the hit with both potential restrictive growing regulations and slowed demand. The lack of progress by the FDA is leading to consumer, manufacturer, and retailer confusion, all of which has needlessly depressed this market. Overall, the hardest hit are consumers who deserve products meeting potency and purity standards as well as proper guidance on how they can gain benefit from these products. We, and others in the hemp industry are urging the regulatory agencies to provide clear and practical regulations to allow the industry to thrive from seed to manufactured products.