Medical marijuana is increasingly being utilized as a holistic treatment for a variety of conditions. While research has been inhibited by the legal status of compounds derived from the cannabis plant, newer scientific and clinical studies are demonstrating efficacy correlating to anecdotal evidence. There are a dramatic number of fewer prescriptions being written per physician in states where medical marijuana is legalized when compared to states where the plant is illegal. Interestingly, the major conditions where marijuana is being utilized are in areas where there are not efficacious pharmaceutical treatments.
Cannabidiol, one of the major cannabinoids found in the industrial hemp plant, has been reported to positively affect a wide range of physiological functions. This non-psychoactive cannabinoid exerts its primary activity as a Non-steroidal anti-inflammatory (NSAID). PANAPure CBD by Panacea Life Sciences and others has demonstrated that CBD is a very good alternative for alleviating pain, demonstrating 40X higher potency than ibuprofen without the addiction potential or other side effects reported for stronger medications such as opiates or gabapentin medications. CBD has also been reported to promote a sense of calm and is being explored as an efficacious anxiolytic and for acute depression [reference]. Equally interesting is that CBD is very well tolerated with minor side effects reported only at extremely high concentrations (>10 mg/kg/day).
The large number of physiological effects attributed to CBD can be explained by interaction with the body’s endocannabinoid system (ECS). While the ECS was discovered a mere 30 years ago, and initially thought to consist only of the cannabinoid 1 and cannabinoid 2 receptors, is now described to consist of over 70 molecular targets that bind to cannabinoids to mediate effects. The collection of molecular targets began to explain how CBD may be an anti-inflammatory, analgesic, anxiolytic, and may provide an alternative to address unmet medical needs without side effects. The role of the ECS is to maintain homeostasis in the body to help stimulate or augment underactive physiological pathways or to help dampen responses for overactive pathways.
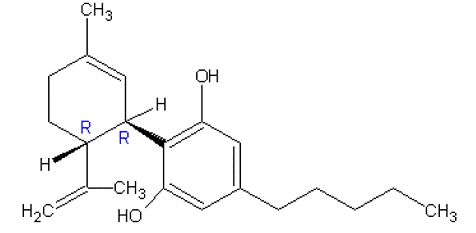
Cannabidiol is a cannabinoid designated chemically as 2-[(1R,6R)-3-Methyl-6-(1-methylethenyl)-2cyclohexen-1-yl]-5-pentyl-1,3-benzenediol (IUPAC/CAS). Its empirical formula is C21H30O2 and its molecular weight is 314.46. The chemical structure is:
Panacea Life SciencesCannabidiol, the active ingredient in Panacea Life Sciences Cannabidiol Oral Solution and EPIDIOLEX, is a cannabinoid that naturally occurs in the Cannabis sativa L. plant. Cannabidiol is a white to pale yellow crystalline solid. It is insoluble in water and is soluble in organic solvents.
Founded in 2017, Panacea Life Sciences was founded to purposely formulate cannabinoids for patients in need and to provide improved scientific/medical knowledge on the benefits of cannabinoid therapy. The hemp industry has been unreliable at best with over 50% of current products available to consumers failing potency and purity quality control checks [reference]. From the first day of operation PLS has instituted unmatched quality processes in supply chain and manufacturing. PLS is completely vertically integrated meaning that the entire process from hemp seed to finished product are owned and controlled by PLS. Our 250 acre farm located in Western Colorado allow PLS to control the quality of industrial hemp used for production as well as access to unique genetics that will be the basis for a next generation of cannabinoid medications. Our 51,000 square foot cGMP facility located in Golden, Colorado focuses on plant extraction, formulation and manufacturing of over 30 products. Manufacturing is quality controlled with extensive testing to ensure that every PLS product will meet or exceed potency and purity standards.
PLS is also committed to advancing cannabinoid science. Leslie Buttorff, Panacea’s CEO, recently donated $1.5M to Colorado State University to fund the CSU Cannabinoid Research Center. This center will serve as the foundation to study various cannabinoids and will expand into various areas of biological research including plant genetics, agronomy, molecular mechanisms of cannabinoid action, as well as animal and human clinical trials. In parallel to CSU research, Panacea has initiated two animal clinical studies to evaluate effects on pain and anxiety in dogs and horses. PLS has launched initiatives to evaluate understanding of cannabinoid activities through scientific collaborations with academic and industrial institutions, as well as Panacea-directed efforts to evaluate the molecular pharmacology of cannabinoids, effects on specific inflammatory pathways, unique anti-infective activities, and determination of anti-tumor activity with linkages of activity to biomarkers associated with tumorgenicity.
Traumatic Brain Injury (TBI)
Traumatic brain injury usually results from a violent blow or jolt to the head or body. An object that penetrates brain tissue, such as a bullet or shattered piece of skull, also can cause traumatic brain injury. Civilian TBI is typically mild that affects brain cells temporarily that often resolves over a three-month period with focus on rest and possible cognitive therapy. Veterans typically experience moderate to severe traumatic brain injuries, with more extreme conditions that include persistent headache pain, seizures, confusion, mood swings, anxiety, depression, sleep disorders, and social isolation amongst a number of other symptoms. TBI patients are also have an increased risk of developing additional neurodegenerative disorders such as Alzheimer’s Disease, Parkinson’s Disease and Dementia pugilistica.
Current treatments for TBI, aside from initial stabilization, are insufficient to specifically assist patients to recover from injury and focus on rehabilitation. A majority of treatment consists of various counseling and psychotherapies. For patients experiencing seizures, anti-seizure medications may be prescribed to control severity and incidence of seizure occurrence.
Over 35% of those with TBI will also suffer from Post Traumatic Stress Disorder (PTSD). PTSD is a mental health condition that results from being exposed to a traumatic event. Patients with TBI and PTSD will present with an additional severity of mental issues including anxiety, depression, sleep disorders, memory issues, and social avoidance. Typical treatment for PTSD involved counseling as well as medications to lessen symptoms of anxiety and depression.
Cannabidiol Oral Solution as a potential alternative treatment for TBI and PTSD?
Recent research has demonstrated that Cannabidiol Oral Solution may be an alternative approach to accelerating healing of the injuries associated with TBI and PTSD (reviewed in LD Schurman and AH Lichtman, Frontiers in Pharmacology 2017 v 8 p 69, online publication; and L Elms J Altern Complement Med 2019 v 4 pp 392-397, respectively). Although CBD, as mentioned above is a potent anti-inflammatory, this compound also exerts neuroprotective activity through various molecular targets including serotonin, dopamine and norepinephrine receptors and transporters. Preclinical research demonstrating CBD efficacy in treating TBI and PTSD have showed promise which has led to two clinical trials to evaluate CBD in TBI (recruiting) and 4 studies to evaluate cannabidiol or marijuana in PTSD patients (including whether CBD will decrease alcohol use disorder).
The best understood and researched activity of CBD is on seizure disorders. There have been over 40 clinical studies evaluating the ability of CBD to lessen the severity and occurrence of seizures with a focus on Lennox-Gastaut and Dravet’s syndrome which are genetic epileptic conditions that cannot be controlled with currently available antiepileptic drugs. GW Pharma has pioneered the development of an FDA approved CBD medication called Epidiolex.
Based on the current research, both preclinical and clinical, CBD holds great promise to alleviate symptoms and possibly treat complex conditions such as TBI and PTSD.
About Epidiolex
Epidiolex, manufactured by GW Pharma, was approved by the FDA September of last year for the treatment of seizures associated with Lennox-Gastaut Syndrome (LGS) or Dravet Syndrome (DS) in patients 2 years of age or older. This medication is a schedule V drug, and as such a prescription is currently required. Although Epidiolex is not approved for conditions other than seizures in these orphan conditions GW Pharma is sponsoring research for off-label prescriptions that would include drug-resistant epilepsy and other seizure disorders.
EPIDIOLEX, cannabidiol oral solution, is a clear, colorless to yellow liquid containing cannabidiol at a concentration of 100 mg/mL. Inactive ingredients include dehydrated alcohol, sesame seed oil, strawberry flavor, and sucralose as sweetener to offset the slightly bitter taste of CBD. EPIDIOLEX contains no ingredient made from a gluten-containing grain (wheat, barley, or rye).
Epidiolex is simple liquid oral formulation consisting of 100mg/ml pure CBD, dehydrated alcohol, sesame seed oil, strawberry flavor and sucralose.
Two randomized, double-blind placebo-controlled clinical studies on Epidiolex efficacy in LGS patients demonstrated a significant decrease in seizure reduction (approximately 40% reduction compared to a 16% reduction in placebo groups) at CBD doses of up to 20 mg/kg/day. Since these patients have severe seizures and are resistant to pharmacotherapy, these results provide a novel approach to those suffering from these two syndromes. Common questions regarding Epidiolex for patients and caregivers is reviewed in R Abu-Sawwa and C Tehling in J Pediatr Ther 2020 v 1 pp.75-77.
There are dose-relate side effects when CBD is taken chronically at doses in excess of 10 mg/kg/day that include excessive sleepiness, decreased appetite, weight loss, diarrhea, transaminase elevation, fatigue, malaise, rash, insomnia, sleep disorder, poor quality sleep, and infections. The transaminase elevation and possible liver damage should be monitored throughout treatment to ensure the continued health of the patient.
Prior to taking Epidiolex, serum transaminases (ALT and AST) and total bilirubin levels should be measured and regularly monitored throughout treatment. Epidiolex has the following contraindications and drug interactions:
- Increased risk of transaminase elevation when taken with Valproate and Clobazam (both are anti-seizure medications).
- Inhibitors or inducers of CYP3A4 and CYP2C19 may require an altered Epidiolex dose.
- UGT1A9, UGT2B7, CYP1A2, CYP2B6, CYP2C8, CYP2C9 and CYP2C19 Substrates may require a reduced dosage when taken with Epidiolex.
Marketing
Epidiolex is an expensive drug that will cost patients approximately $32,000 annually. It is unknown at this time whether insurance carriers or Medicare will cover this medication. The pricing is driven by the drug approval pathway and the limited number of patients.
While FDA approved generic medications are in development, it is not expected that these generic equivalents will enter the market until 2025. Generic equivalents of Epidiolex are anticipated to reduce costs to approximately $6,400 per patient annually.
Tilray 2:100 Tincture: Comparable Product and Results to Epidiolex
Tilray’s 2:100 (THC:CBD) oral solution is a comparable product to Epidiolex. While not a generic drug, it is essentially the same formulation: 100 mg/mL Cannabidiol solution with Sesame Oil, Alcohol, Strawberry Flavor, and Sucralose. This medication is only currently available to medical marijuana patients in Canada. Tilray has not conducted their own clinical trials or research to support the efficacy of their product for the disorders treated with Epidiolex. Furthermore, they do not make any medical claims about the product. Tilray has provided their 2:100 tincture for use in clinical trials in children with Dravet Syndrome; however, these studies pre-date FDA approval of Epidiolex. They have also provided CBD to American universities for several other studies, including:
- PTSD and Alcoholism at NYU (https://www.cnbc.com/2019/08/08/pot-company-tilray-to-import-cbd-for-nyu-clinical-trials.html)
- Severe Behavioral Problems (SBP) in pediatric patients with Intellectual Disabilities (ID) at Murdoch Children’s Research institute (https://www.tilray.com/tilraynews-master/2019/4/1/tilray-announces-support-of-two-new-clinical-research-studies-in-australia-and-canada)
- Reduce chronic immune activation in People Living with HIV, THC+CBD caps (might not be relevant due to combination with THC, link is same as previous).
- Tilray 2:100 is a Canadian product with 100mg/ml CBD and 2mg/ml THC. This is in a coconut oil base produced by cold ethanol extraction methods and filtration.
Panacea’s Cannabinoid Oral Solution Tinctures
Panacea Life Sciences currently manufactures a variety of products, including tinctures, containing various concentrations and preparations of CBD. Since Epidiolex is an extremely simple formula, Panacea has formulated and manufactured a Cannabinoid Oral Solution tincture similar to Epidiolex that is available to consumers at a fraction of the cost. Panacea manufactures and markets these products as dietary supplements with strict quality control so ensure potency and purity requirements are met with each batch produced.
This approach allows Panacea to meet its mission with providing affordable and needed CBD products to consumers with unmet medical needs.
Additional Sources:
https://www.fda.gov/drugs/questions-answers/generic-drugs-questions-answers
https://patentimages.storage.googleapis.com/e8/e5/0c/4b28fa4351a0f6/US9956185.pdf
https://www.drugs.com/availability/generic-epidiolex.html