WHAT RESEARCH CURRENTLY EXISTS AROUND CBD?
Because CBD has only recently entered the medical mainstream, there are still many unanswered questions to be explored. Early research focused on the role of cannabinoid receptors in the brain in perceiving and processing CBD, as well as its effect on the brain’s production of dopamine.
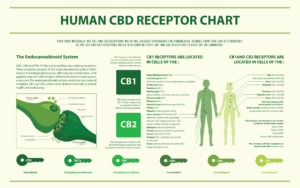
Over the last few years, doctors have launched small-scale CBD studies and CBD clinical trials to find other potential uses for the compound. We DO know that CBD CBD interacts with neuroreceptors in the body’s endocannabinoid system, which sends signals between your cells to help regulate your movement, mood, homeostasis and immune system. This means CBD has major potential for treating a variety of disorders and ailments.
In just a few short years, there have already been hundreds of studies exploring the benefits that CBD can provide. Namely, CBD has been proven to help treat epilepsy, offset anxiety and depression, treat opioid addiction, alleviate unmanageable pain, protect against neurological diseases, and inhibit arthritis symptoms.*
CURRENT & RECENT CBD CLINICAL TRIALS
The following CBD trials are the most up to date known to ClinicalTrials.gov.