Topical products containing cannabidiol (CBD) are proving to be one of the fastest-growing trends within the hemp market. Of the roughly 1.2 billion dollar CBD market in 2019, $736 million is comprised of CBD topicals. Even more exciting — the topical market is expected to surpass 3.8 billion dollars by 2026, with a staggering 24% CAGR. The current market is fragmented with boutique topical manufacturers offering products, and a small but growing number of retailers offering CBD products. Currently, Kroger, Walgreens, CVS Pharmacies, and Rite Aid have engaged in carrying CBD products. Specialty stores such as Ulta and GNC are focusing on specific CBD products for their customer base. These products are popular for consumers and retailers alike, namely due to their ease of use, benefits to the skin, and clear guidance by the Food and Drug Administration (FDA) regarding product legality.
Legalization Loopholes
A significant issue for retailers that are considering carrying any CBD product is whether the products are legal to be manufactured and sold. However, one of the reasons for the surge in topical CBD sales is the clear federal legality and regulatory pathway.

Cannabidiol derived from industrial hemp was federally legalized with the passing of the 2018 Agriculture Improvement Act, commonly referred to as the Farm Bill. From that point, many uses of CBD are under evaluation by the FDA to test for safety and to gain clarity about how CBD products should be regulated.
While ingestible cannabidiol is under evaluation, the FDA made it very clear that under the Food, Drug, and Cosmetic Act, cosmetic products and ingredients are not subject to premarket approval by FDA (except for most color additives). Certain cosmetic ingredients are prohibited or restricted by regulation, but currently that is not the case for any cannabis or cannabis-derived ingredients.
Although federally legal, there are a few states (currently Idaho, Nebraska, and South Dakota) that still prohibit CBD products, in which retailers are encouraged to evaluate prior to placing CBD products on their shelves.
Great! So, what’s the problem?
While the cosmetic industry seems to be in the clear for manufacturing and selling CBD products, due to the uncertainty in regulations regarding most CBD products, there is a lack of ingredient identity and conformity for most CBD products. Cannabidiol is extracted from the industrial hemp plant and may be used as an ingredient in few forms:
- Full-spectrum oils contain all the components of the plant, including terpenes, which give the hemp plant its characteristic cannabis smell, among other cannabinoids.
- Broad-spectrum oils are refined to contain all the components of full-spectrum oil, but the tetrahydrocannabinol (THC — the psychogenic component of cannabis plants) has been removed.
- CBD isolate is pure CBD.
Based on the desired effect of the CBD ingredient, manufacturers may choose a full or broad-spectrum oil or stick with pure CBD. In order to correct the lacking uniformity, the Personal Care Products Council has taken a lead to adopt nomenclature that provides much-needed clarity for cannabidiol producers, personal care product manufacturers, retailers and consumers. The names designated by the International Nomenclature Cosmetic Ingredient (INCI) describe the strength of the CBD in the oil ingredient as well as its level of refinement.
Are CBD’s benefits worth the legal ambiguity?
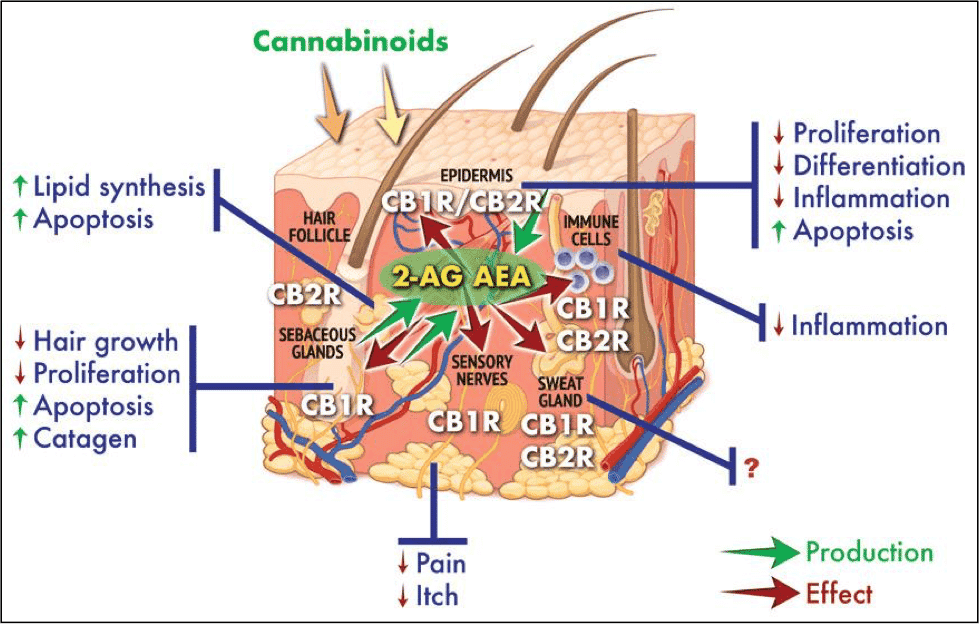
Most topical market players would argue yes. Cannabidiol works through the endocannabinoid system (a diverse collection of enzymes, ion channels, and receptors in the body) to exert primarily anti-oxidative and anti-inflammatory activities. Inflammation is a catch-all phrase for how the body reacts to and combats insults to the body, whether it be a damaged body part (e.g. sprained ankle), infectious agent, or uncontrolled cell growth like that of psoriasis or cancer. Because of CBD’s anti-inflammatory activity, this cannabinoid has been reported to treat or alleviate a wide array of conditions, from bacterial infections to arthritis pain (S.A. et al., Antioxidants 2020 v9 p21).
Additionally, CBD and CBD hemp oils are easy to formulate into topical products. CBD products range from essential oil-based salves to lotions, lip balms, shampoos, and bath bombs. Manufacturers will most likely make numerous claims about CBD’s ability to ease stress, improve skin health, and assist with work out recovery. While some of these claims are hopeful at best, there is a growing body of scientific studies that support many positive health impacts that topical CBD products provide.
Over the past few years, since the passing of the 2018 Farm Bill, research on the biological effects of CBD has increased dramatically. Cannabidiol has been reported be very well tolerated whether ingested or applied topically. CBD will absorb into the skin, and unless specifically formulated, will not pass through the skin into the blood stream. Thus, topical formulas of CBD are generally concentrated at the site of application. In fact, CBD’s anti-inflammatory properties have been reported to provide benefit for many skin conditions:
Acne: The skin disease with complex pathogeny with inflammation at its center. Acne is triggered by various processes such as excessive oil production by sebocytes, hormonal imbalances, immune reactions, and microbial infections, as well as other factors. CBD has been shown to address underlying inflammation associated with acne but also decreases sebocyte production of oil and inhibits bacterial growth. A phase 2 clinical trial — focused on the ability of a CBD preparation to treat acne and scaring — has recently been completed with results pending. The potential ability of CBD to address several aspects of acne make this a promising ingredient to address this condition.
Anti-infective: CBD has recently been reported to have anti-microbial activity, specifically working on antibiotic-resistant strains of bacteria (including methicillin resistant Staphylococcus aureus), and working in concert with existing antibiotics (2019 American Society for Microbiology Microbe, Dr. Mark Blaskovich and University of Southern Denmark ). These results suggest that CBD should be incorporated not only into acne creams, but also into topical sanitizers and soaps.
Allergic Contact Dermatitis: This condition is a delayed hyper activation of the immune system when the skin comes into contact with an allergen. CBD has been shown to inhibit this inflammatory reaction which suggests that CBD lotions may alleviate this dermatitis and potentially enhance “allergen-free” topical lotions and detergents.
Psoriasis: This autoimmune disorder causes keratinocytes to hyper-proliferate, resulting in rashes or plagues of gray, scaly skin. This condition is also associated with pain. Psoriasis has been shown to be caused by a dysregulation of the immune system, so current pharmaceutical therapies target the proteins released by immune cells that result in epidermal proliferation. CBD has been shown to directly inhibit proliferation of keratinocytes while also lessening the associated inflammation.
Arthritis Pain: Arthritis is a condition in which the inflammation of joints leads to swelling, pain, stiffness, and diminished motion. Topical administration of CBD has been shown to reduce inflammation and pain in preclinical models by as much as 50% after four days of treatment. Additionally, there are numerous anecdotal reports that topical administration reduces the swelling in joints, increases joint mobility and decreases pain. More defined clinical studies are needed to determine correct dosing and the extent that CBD may alleviate this condition.
Skin Aging: CBD has greater antioxidant activity than the better-known vitamins C and E. Through this activity, CBD can prevent damage to skin cells and promote recovery to healthier-looking skin. CBD has also been shown to stimulate the synthesis of collagen to help maintain skin smoothness and elasticity, which in turn is proposed to reduce wrinkling and sagging skin.
Hair loss: Scalp inflammation has been proposed to be a cause of alopecia or hair loss. A 2019 clinical trial found that commercial hemp-oil products boosted hair growth over a six week period. Notably, the hemp oil used was CBD oil, not hemp seed oil, the latter of which does not contain cannabidiol. The hypothesis is that CBD improves blood circulation to improve the health of hair cells while protecting against damaging inflammatory responses, similar to the way it interacts with psoriasis.
With such a well-tolerated ingredient that has multiple skin health benefits, it is easy to understand consumer excitement and demand for these products. After looking at the multitude of benefits provided by the inclusion of CBD, it is only logical to conclude that more and more retailers and consumers will want to add these products to their shelves and shopping lists.